Isotopes, isobars and isotones
Isotopes
The atoms of same element having same numbers of protons in nucleus but different numbers of neutrons are called isotopes.
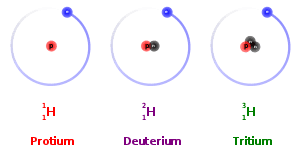
Hydrogen has three isotopes such as H-1, H-2, H-3. In all of these isotopes number of proton and electron are same as one while number of neutron differ, protium has no neutron, deutrium has one and tritium has two neutron as shown below.
Number of isotopes vary for an element some element has one and some have more than one which may be two, three, four, five, six etc.
Isotones
The atomic species having same numbers of neutron but different numbers of protons are called isotones.
- Carbon C-14 and nitrogen N-15 are called isotones as they have same number of neutron in their nucleus
Isobars
The atomic species having different atomic number but same mass number are called isobars
- Potassium K-40 and calcium Ca-40





Comments
Post a Comment
If you have any doubt, let me know.